This is a part of the lecture notes in process control.
Example of a dynamical process
In this lecture, we will see how to create mathematical description of dynamical processes. The first example will concern a mixed tank heated up by water steam. This device is shown in Fig. 1. The tank is continuously supplied with a stream of liquid in amount of FV (volumetric flow rate in m3/s). The temperature of the liquid at the inflow equals Tf (further subscript f will often be used to denote ‘feed’). The tank is equipped with a heating jacked, where the hot steam is supplied. Through the walls of the tank, heat is transferred to the liquid inside of it. Therefore, the temperature of the liquid will rise to certain value T. Due to the agitator inside the tank, the temperature is the same at every point of the liquid. At the bottom of the tank, the liquid flows out. The temperature of this stream is the same as the temperature inside the tank, that is T.
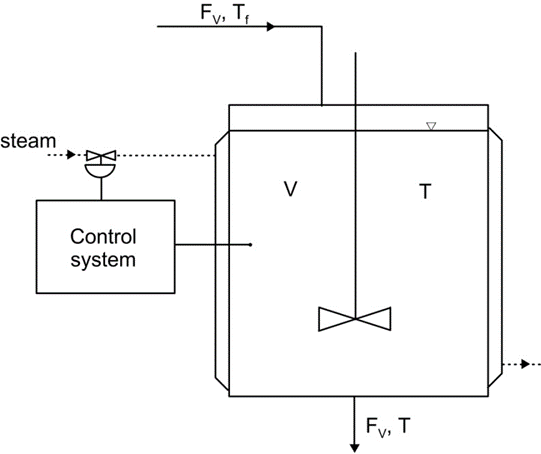
The most simple way to control the temperature is to use so-called bang-bang controller (or on-off controller). The idea of such approach is that when the temperature T decreases below some assumed value, then the valve supplying the heating steam is opened. When the temperature increases above other assumed value, the steam supply is closed. In order to say how the temperature changes, we need to formulate a mathematical model of the device. To this end a heat balance should be formulated.
How to formulate a dynamical model?
The heat balance (as other balance equations) is formulated based on two rules. First, we need to specify the volume of balance, also called a control volume. When one deals with objects as tank with agitator, usually the control volume is equal to the volume inside the tank. Such situation takes place, when the temperature (or other important quantities) are uniform inside the medium. In this case, the temperature is uniform in the whole volume, therefore the control volume is V. Secondly, we need to define the terms which should be taken into account. Each term in the balance equation correspond to one factor (or phenomenon) that influences the amount of heat in the control volume.
There are three terms that should be included in the heat balance. Because, the tank is supplied with a liquid of temperature Tf it also contains thermal energy. This term will be called ‘convective inflow rate of heat’. Due to the same reason, there will be also ‘convective outflow rate of heat’. The last term is the heat transfer from the steam to the liquid inside the tank. All terms added together give ‘heat accumulation rate in the control volume’. Each term has a corresponding sign indicating the increase or decrease of heat amount. The above reasoning is summarized by the following equation:
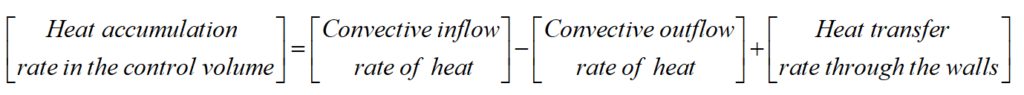
Dynamical model of a heating tank
In order to obtain any quantitative results we have to introduce mathematical description. As a rule, each term in the heat balance equation is in units of J/s (heat by the unit of time, not necessarily in Joules per seconds). The equation takes the following form:
(1) ![Rendered by QuickLaTeX.com \[(\underbrace{V\cdot \rho \cdot {{c}_{p}}}_{\begin{smallmatrix} heat\ capacity \\ of\ liquid \end{smallmatrix}}+\underbrace{{{m}_{s}}{{c}_{s}}}_{\begin{smallmatrix} heat\ capacity \\ of\ solid \end{smallmatrix}})\frac{dT}{dt}={{F}_{V}}\cdot \rho \cdot {{c}_{p}}\cdot {{T}_{f}}-{{F}_{V}}\cdot \rho \cdot {{c}_{p}}\cdot T+{{A}_{q}}{{k}_{q}}({{T}_{q}}-T)\quad \left[ \frac{J}{s} \right] \]](https://softinery.com/wp-content/ql-cache/quicklatex.com-b7ef166bdb0095ae6b86fdc305eba27f_l3.png)
where:
V – volume, m3
ρ – density, kg/m3
cp – specific heat of the liquid, J/(kgK)
Aq – area of heat transfer, m2
kq – heat transfer coefficient, J/(m2sK)
Tq – temperature of water steam, K
At the initial time, the temperature inside the tank is equal:
(2) ![]()
The accumulation rate is calculated as the product of the derivative dT/dt and heat capacity – the term in the parentheses. Heat capacity consists of heat capacity of the liquid and heat capacity of the solid material in the tank (for example agitator).
The Eq. (1) can be transformed to:
(3) ![]()
where:
![]() ,
, ![]() ,
, ![]()
As was stated above, the device is controlled by on-off control. Therefore we can distinguish two phases of operation: the heating phase and the cooling phase. The heating phase is described by the Eq. (3). The heating phase starts when the temperature decreases below an assumed value. We will denote it by Tlow, so at the initial time:
(4) ![]()
The equation describing cooling phase is derived in the same way. However, because there is no heat transfer due to lack of steam supply, the last term of the heat balance equation is equal zero. After transformations, we will obtain:
(5) ![]()
The cooling phase starts when the temperature exceeds an assumed value. We will denote it by Thigh, so:
(6) ![]()
Solution of a dynamical model
Eqs. (3) and (5) are used to obtain quantitative description of the device operation. To this end it is required to solve them. The procedure will be described on exercises. The solution is following:
– heating phase:
(7) ![]()
– cooling phase:
(8) ![]()